
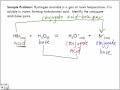









Which one of the following correctly represents a conjugate acid-base pair? H2CO3, HCO3 If the hydronium ion concentration of an aqueous solution at 25°C is 5 × 10−6 M, what is the hydroxide ion concentration? There is no such ion as H2CO3- However, the neutral molecule H2CO3 exists. Its conjugate base is the bicarbonate, or hydrogen carbonate ion: HCO3- The conjugate base of the bicarbonate ion is the Solution for Which one of the following pairs is a conjugate acid-base pair? H2CO3, CO32- H2CO3, HCO3- HCl, Cl H2O, O- H3O+, OH- For a, the acid is H2CO3, and the base is water. During the acid-base reaction, you remove a proton (or hydrogen atom) from H2CO3, and attach it to H2O. Therefore H2CO3 becomes HCO3-, and H2O... So once the base has accepted a proton it is an acid. We call this the conjugate acid of the base. OH- + H+ -----> H2O. base + proton -----> acid. conjugate pair is. OH- / H2O. A stong acid has a... The conjugate base contains one less H than its corresponding acid and 1 more negative charge than its corresponding acid. So, the conjugate base for H2CO3 H 2 C O 3 HCO− 3 H C O 3 −. TABLE OF CONJUGATE ACID-BASE PAIRS Acid Base K a (25 oC) HClO 4 ClO 4 – H 2 SO 4 HSO 4 – HCl Cl– HNO 3 NO 3 – H 3 O + H 2 O H 2 CrO 4 HCrO 4 – 1.8 x 10–1 H 2 C 2 O 4 (oxalic acid) HC 2 O 4 – 5.90 x 10–2 [H 2 SO 3] = SO 2 (aq) + H2 O HSO The conjugate base of H 2 CO 3 is HCO 3 - . To determine the conjugate base, remove a proton (H +) from the acid. The formula will have one less hydrogen... See full answer below. acid base base acid. here H2CO3 and HCO3- are conjugate acid-base pair as are H2O and OH-b) HCl + H 2 PO 4-<-----> Cl - + H 3 PO 4. HCl transfers a proton (H+ ion) to H 2 PO 4- and form Cl - and H 3 PO 4, now Cl - can accept a proton donated by H 3 PO 4 .so the above equation is; The conjugate base of bicarbonate, HCO 3- is carbonate, CO3 2-. HCO3- is a conjugate acid, H 2 CO 3. Log in or register to post comments; Similar Questions. What is the conjugate acid of base HCO3- ? The Ka for HCO3- is 4.7 x 10^-11, what is the conjugate base and its Kb?
[index] [3670] [7437] [2925] [1855] [1550] [1680] [6313] [6211] [373] [3947]
In the Brønsted-Lowry definition of acids and bases, a conjugate acid-base pair consists of two substances that differ only by the presence of a proton (H⁺).... About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ... Identify the acid, base, conjugate acid, and conjugate base of acetic acid reacting with water: CH3COOH (aq) + H2O (l) ⇌ CH3COO- (aq) + H3O+ (aq) This chemistry video tutorial explains the concept of acids and bases using the arrhenius definition, bronsted - lowry and lewis acid base definition. It al... Introduction to conjugate acids and bases. Created by Sal Khan.Chemistry on Khan Academy: Did you know that everything is made out of chemicals? Chemistry is...
Copyright © 2024 m.sportbetbonus772.ink